VTA Insider June 2024: VTA Testifying before congress, Update on the state of States

Published on June 26, 2024
Welcome to the VTA Insider, your industry focused briefing from the Vapor Technology Association.
The VTA Insider will keep you informed of critical issues impacting the vaping industry both in Washington, DC and in the states. We will cover regulatory developments, legislation, news of note and other industry insights. VTA will also share with you our own analysis of what we as an industry need to do to grow and prosper.
Lets Dive In.
VTA testifies before Congress
On Wednesday, June 12, 2024, VTA’s Executive Director Tony Abboud had the unique opportunity to testify before the Senate Judiciary Committee in Washington, D.C. to address the myriad problems with FDA’s regulatory approach to e-cigarettes. Abboud’s testimony provided the Committee with critical scientific data shared tobacco-control scientists’ calls for a dramatic change in U.S. regulators’ approach to e-cigarettes.
What we’re saying: Abboud’s testimony touched on three key points:
- FDA’s many failures and the hypocrisy of the leading health regulator authorizing 821 new cigarettes and ZERO less harmful vaping or pouch products in the last two years.
- The facts regarding the dramatically ameliorated youth vaping rates in the U.S. coupled with an urgent message to Congress to take up the long overdue marketing and access restrictions that VTA has been advocating for since 2018 to actually protect youth.
- A renewed our call for the FDA to reverse course, authorize a wide variety of flavored e-cigarettes, make harm reduction its north star to encourage Americans who smoke to try e-cigarettes, and to enforce the Tobacco Control Act in a manner that will give tens of millions of Americans a regulated choice of less harmful flavored nicotine options and a fighting chance to get off deadly cigarettes.
Go deeper: You can catch the full hearing testimony here and read a transcript of Mr. Abboud’s opening testimony here.
UPDATED: State of the states
State of the States: Most state legislative sessions have adjourned till the fall, but there are still a few PMTA registry bills working their way through:
- In North Carolina, PMTA registry language was snuck into H900 without any hearing or public input on the matter by big lobbyists activists. If you live / work in North Carolina, please use and share our call to action to help stop this pernicious and desperate tobacco ploy today. Your representatives need to hear from you.
- In Louisiana, HB 621 updating the state’s PMTA registry bill passed both houses of the state legislature and was signed by Gov. Jeff Landry.
- In Rhode Island. Gov. Dan McKee signed the state budget bill H 7225, converting the health department’s flavor ban to a state law.
- In Iowa, Gov. Kim Reynolds signed HF 2677 into law, beginning the process of creating a PMTA registry in Iowa later this fall.
What’s next: Our fight against these bills is not over.
- We expect a few more states to address PMTA registration bills this year and we expect new and renewed attempts to create or expand PMTA registries next year.
- VTA is hard at work educating on the public health and small business damage these bills would create.
Vaping and Chevron deference

This week or next, the United States Supreme Court will release its remaining opinions of the term. The Court typically waits until final week to release to “big” decisions and there is none bigger than Loper Bright v. Raimando, the case that challenges the long standing doctrine known as Chevron deference.
Why it matters: Chevron deference provides federal agencies with the ability to interpret and implement ambiguous statutes, so long as these interpretations are “reasonable.” For years, the doctrine has been used to uphold federal agency actions even when they seem to exceed statutory authority.
For the vaping industry that has meant:
- FDA’s arbitrary and capricious vaping policies have been protected by Chevron deference.
- The vaping industry has been forced to bow to the political whims of an agency that does not want to approve less harmful non-combustible nicotine products.
What’s Next: Some legal analysts have opined that the Supreme Court could overturn or severely restrict Chevron deference and others have opined that the Court could narrowly restrict its ruling to the facts of the case.
- If the Court does the former, we see an avenue to force the FDA to fix their infamous “fatal flaw” approach that was designed to quickly deny marketing authorization for as many non-tobacco flavored ENDS as possible and start relying on science instead of politics in the PMTA process.
Flavored vapes for thee, but not for me

Last week, in a first, the FDA authorized menthol flavored vaping devices and finally acknowledged the enormous body of science proving flavored e-cigarettes help Americans quit smoking – not to mention FDA’s first authorization for a flavored disposable product at the highest nicotine concentration available on the market.
- The catch is that, to date, the only vaping products authorized are those manufactured by Big Tobacco companies.
- The second catch is that the week prior, FDA denied several other applications from small vaping maufacturers and wants to keep their products off the market.
Why it matters: While the news is a tiny step in the right direction, it also reveals a more troubling pattern:
- FDA is acting only in self-interest to quell political pressure rather than acting in the interest of the American people.
- Approving some but denying other substantially similar products is going to bring renewed scrutiny on how FDA is arbitrarily applying its it’s public health standard which, to date, it has refused to clearly articulate.
The bottom line: The only vapes authorized today are all owned by the biggest cigarette companies in the world, demonstrating Brian King and the FDA’s hypocritical allegiance to those companies whose deadly cigarettes and other combustible products the FDA continues to flood the market with at a record pace (more below).
E-cigarettes are the most effective smoking cessation tool on the market and are at least 95% safer than combustible cigarettes. It is far past time for this FDA to approve a wide variety of flavored e- cigarettes and stop authorizing dangerous cigarettes.
FDA’s tawdry Big Tobacco record
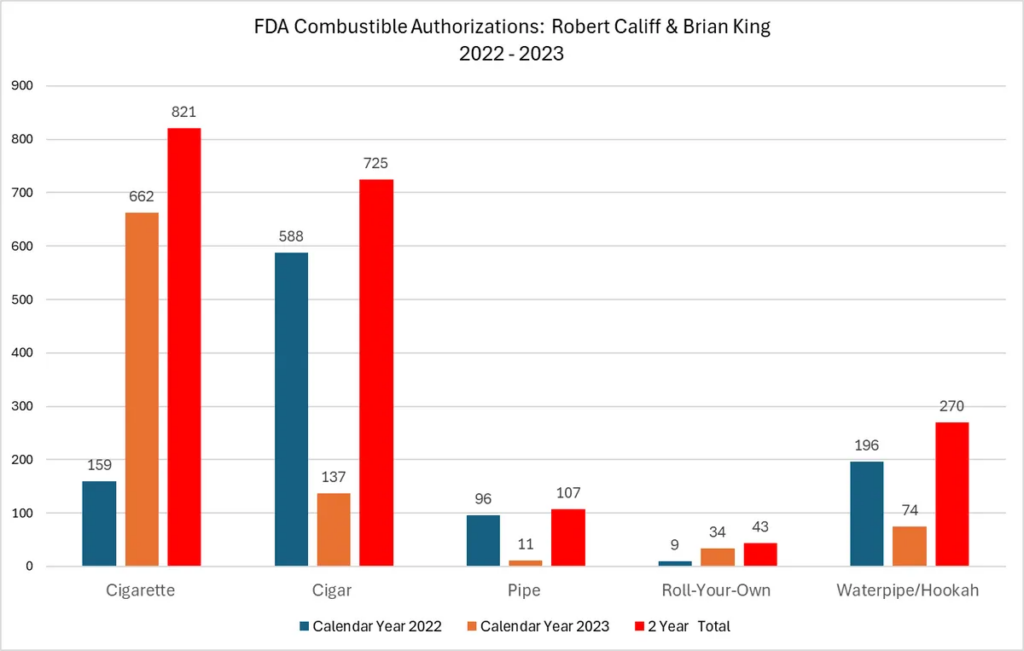
Last year, VTA did a deep dive into FDA’s tobacco product approvals. The numbers were shocking then, and things have only gotten worse as our updated May 2024 report FDA is on Fire proves.
Why it matters: Until last week, FDA has refused to authorize a single premarket tobacco application for less harmful electronic nicotine delivery systems. And they have refused to authorize a single less harmful modern oral nicotine product.
What we found: In stark contrast to the more than 16,800 combustible products that FDA has authorized from 2009 to December 2023, the FDA has seen fit to authorize a paltry 45 next generation tobacco products. By category, FDA’s 45 authorizations include:
- 2 new combustible cigarettes (low nicotine, but still combustible)
- 4 oral tobacco mints and chews
- 8 smokeless tobacco products (snus)
- 8 heat-not-burn products (the IQOS device and assorted Marlboro HeatSticks)
- 23* e-cigarettes (out of more than 20M applications filed).
*Since facts do matter, it is important to note that the often-cited list of “23” authorized e-cigarettes, only includes about 8 unique e-cigarette devices (some of which are no longer available on the market). The remainder of the list of 23 “e-cigarettes” are either replacement pods, accessories, or other products not traditionally identified as e-cigarettes.
You can read VTA’s Report – FDA is on Fire here.
Post script: With the new menthol authorizations, the net available vape devices on the U.S. market now hovers around 10.
The FDA enforcement trap

The latest: At last month’s Food and Drug Law Institute’s Tobacco & Nicotine Policy Conference in Washington, D.C., VTA Executive Director Tony Abboud presented thoughts on FDA’s broken enforcement policies and what that means for the future of tobacco product regulation.
Go Deeper: Tony’s presentation highlighted some of the major problems that that FDA has created with its misguided policies in pretending that it can enforce prohibition:
- FDA policies have created a de facto ban on ENDS products resulting in ZERO product approvals during the current commissioner’s tenure;
- FDA’s enforcement of ENDS devices is not viable or efficient and is helping to create illicit markets;
- FDA’s enforcement policies are misplaced given that there are three times the number of youth violations for combustible tobacco products vs. vaping products;
- FDA’s flavor ban enforcement is driving smokers back to cigarettes
You can view a copy of Tony’s presentation here.
Worth Your Time

- VTA Executive Director Tony Abboud joins Fox News to discuss FDA’s failures and need for flavored e-cigarettes
- VTA Executive Director Tony Abboud quoted in Washington Post story
- Vape bans could lead to smoking comeback among children
- Phillip Morris suspends Zyn online sales nationwide after DC subpoena
Stay tuned in with us for lots of new developments and make sure your friends and colleagues are signed up to receive our news and information.
Got questions? If you have any questions about the issues we have covered, have suggestions for content, or how you can support our efforts, please feel free to contact us at press@vaportechnology.org.