VTA Insider May 2023: LBGTQ & Nicotine Products

Published on May 30, 2023
VTA Insider keeps you informed of critical issues impacting the nicotine products industry both in Washington, DC and in the states. We will cover regulatory developments, legislation, news of note and other industry insights. VTA will also share our analysis of what we as an industry need to do to grow and prosper.
Let’s Dive In.
A heavy lift in DC

The big picture: The E-Cig Summit and the Food and Drug Law Institute convened in Washington, D.C. There is too much to detail in this newsletter but a few key big-picture takeaways:
Science is clear: Scientists from around the world convened to discuss the continuing growing body of the science behind the importance of vaping and nicotine alternatives to smoking cigarettes. In study after study presented, the scientists presented unflinching perspectives on everything from data to policy.
The Consensus: FDA is broken and is failing in delivering an effective harm reduction message. Vaping can be a huge benefit to public health and there is ample evidence supporting the potential of vaping to reduce smoking’s toll. Making it harder for adults to access vapor products makes it harder for them quit and ensures that they engage in the riskiest behavior, (smoking) longer.
Why it matters: Litigation is now effectively driving health policy. FDA had a plan to review the large number of PMTAs received and to streamline its reviews in a scientifically defensible matter for flavored ENDS. FDA had announced its plans, priorities and processes in June 2021.
- Then, all that changed with the direct interference from a former Acting Commissioner who forced the creation of the “fatal flaw” memorandum which was used to en masse deny all flavored vaping products.
- This and other interference with scientific decisions was called out by scientists inside & outside FDA’s Center for Tobacco Products.
- Given FDA’s actions, companies had no option but to litigate.
FDA enforcement needs to be focused on the bad actors, i.e., those companies which have completely ignored FDA’s regulations. While Director Brian King proudly spoke about the pace of PMTA denials (99% of all vaping products) he acknowledged the difficulty with enforcement. No surprise here since bans cannot be enforced. But, as was highlighted in the Reagan-Udall report, FDA has taken little action against companies that chose never to even apply through the PMTA process.
Misinformation for the masses
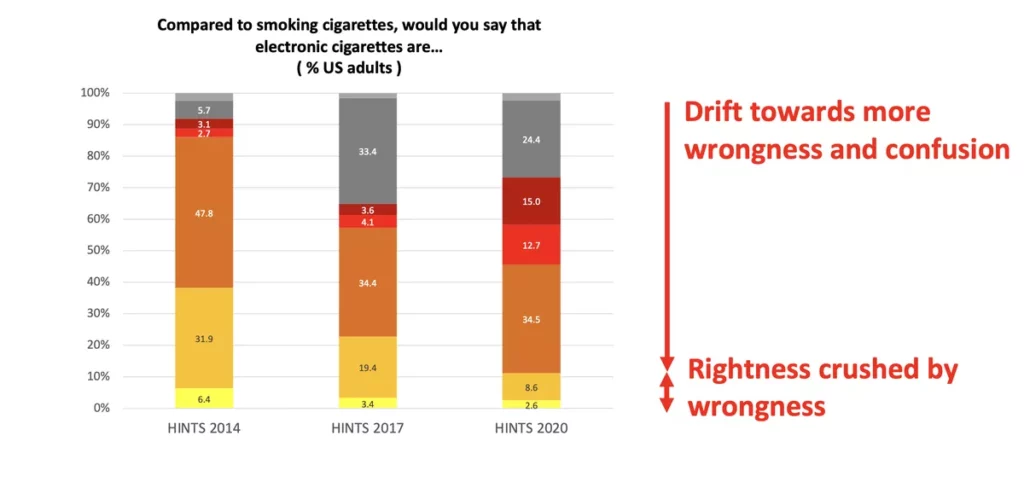
At the E-Cig summit last week, our friend Clive Bates of Counterfactual gave an excellent presentation focused on how the U.S. should be rethinking tobacco and nicotine regulation. The entire presentation is worth a read but the chart above is a perfect encapsulation of the problem.
The public is being fed misinformation concerning the relative health risks of smoking vs. vaping and the problem is getting worse. People are now more likely to believe that vaping is as dangerous as smoking than they were ten years ago.
FDA officials acknowledged the problem of misinformation during the Q&A segment at the E-Cig Summit and committed to developing a public affairs campaign targeted at adults. These type of campaigns take months to develop so don’t expect anything dramatic anytime soon.
Doubling Down on Youth. In the same talk, however, CTP Director Brian King, who again acknowledged the dramatic decline in youth vaping, doubled down on youth experiementation. He said there is no acceptable level of youth vaping usage, foretelling what will continue to dictate FDA’s continued vaping product denials.
The bottom line: Quitting smoking is hard. Only 10% of adults who try to quit will ultimately succeed. FDA needs to expand the understanding of the dramatically less risky alternatives to smoking for tens of millions of adults who are addicted. Of course vaping is not free from risk and there are trade-offs but those tradeoffs should be made explicit so that we can reduce death and disease by giving people the information to de-risk their behavior.
- But, Director King went on to say that there was a difference between enforcement discretion and enforcement prioritization.
- King promised promised a formal letter soon from CTP in response to VTA’s questions to clarify CTP’s position.
Advancing harm reduction equity
The big picture: CTP Director Brian King has made the concept of “health equity” as it relates to tobacco product regulation a centerpiece of his plan. King characterized FDA’s focus on health equity in the following way:
- “We have a tremendous opportunity to prioritize health equity and create meaningful change for populations that have been disproportionately affected by tobacco use.”
- Director King also created the first “health equity” leadership position inside CTP.
By the numbers: Smoking remains the number one health risk to adults in the United States. Approximately 34 million adults are still addicted to cigarettes – the only product which when used as intended will kill you. In the LBGTQ communities – the problem is worse. LGBTQ people out-smoke the general population by 68%. This high rate of LGBTQ smoking is a current and ongoing health crisis. In fact, according to the NIH, smoking related deaths are on track to surpass HIV/AIDS as a cause of death among gay men.
Why it matters: CTP is promoting health inequity and would be better served to focus on vaping as a tool to promote “harm reduction equity” which would have more immediate benefits and potentially immeasurable benefits for historically marginalized communities.
The bottom line: The FDA and its Center for Tobacco Products (CTP) is charged with protecting all Americans from tobacco-related disease and death.
- This means the FDA must do everything to help people move off combustible tobacco and toward lower risk alternatives. FDA’s backward approach to harm reduction means that it is easier to get a new deadly cigarette product approved than an e-cigarette alternative.
- And the fact that the FDA has declared more than 99% of vaping products “illegal” means that the only choice left remaining will be deadly cigarettes.
Harm Reduction Equity: Conversely, a focus on harm reduction equity means telling the communities that are disproportionately impacted by smoking related death and disease the truth – that vaping while not risk free is less risky than smoking. Given the unequal burden the LGBTQ community and other populations disproportionately affected by smoking (such as the African-American community, those of lower incomes, those afflicted with mental health issues) bear for cancer and other health risks associated with smoking, limiting adult choices and access to lower risk products will only serve to expand health inequities in our society.
The real story on combustibles

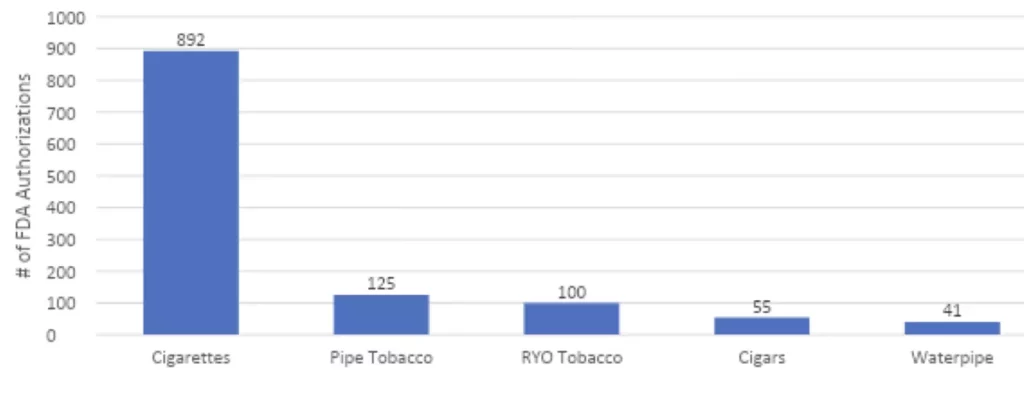
FDA has a responsibility to create a marketplace that allows the most innovative and safe products to emerge so that adults have access to them. With all the news about the product denials for flavored vaping products, you might be surprised to learn that the FDA has approved for sale more than 1,200 combustible products including nearly 900 new cigarettes!
FDA Approvals of Combustible Tobacco Products 2018 –2022
FDA Commissioner Robert Califf recently said in a March 6, 2023 Wall Street Journal interview, “There aren’t many people who argue in favor of combustible tobacco … Well, I mean, we always start from the presumption that combustible tobacco is the most dangerous part of this. And we need to get rid of that as much as we possibly can. It’s written into the mission of the FDA.”
There aren’t many people who would argue with that. So why is the FDA under his leadership approving so many of combustible products? You can check out the full VTA analysis of FDA product approval data here.
FDA’s continued refusal to accept known scientific truths about vaping and modern oral nicotine will only ensure that cigarettes remain dominant in the U.S.
Worth your time

Leading Tobacco-Control Experts call on FDA to change – and fast.
- some of the world’s leading tobacco-control scientists and public health experts wrote to FDA Commissioner Califf calling on him and the FDA to rethink its current posture.
- The group focused on the hyper-critical findings of the Reagan-Udall Foundation’s review of CTP and made specific recommendations for changing the way CTP handles tobacco and vaping regulations.
Juul Whitepaper: Juul Labs Inc. has published “The Real-World Impact of ENDS for Adult Smokers: Tobacco Harm Reduction Through Real-World Data and Evidence.” We encourage you to read it here.
Two main requirements of effective harm reduction: (1) that the products are much less harmful than cigarettes, and (2) that these products displace smoking at the population level.
No doubt the whitepaper it will be panned because of the source but “risk-proportionate regulation” is related to FDA’s “continuum of risk” concept. We have to keep pushing to embed this harm reduction priority as our public health strategy.
Stay tuned in with us for lots of new developments and make sure your friends and colleagues are signed up to receive our news and information.
Got questions? If you have any questions about the issues we have covered, have suggestions for content, or how you can support our efforts, please feel free to contact us at press@vaportechnology.org.