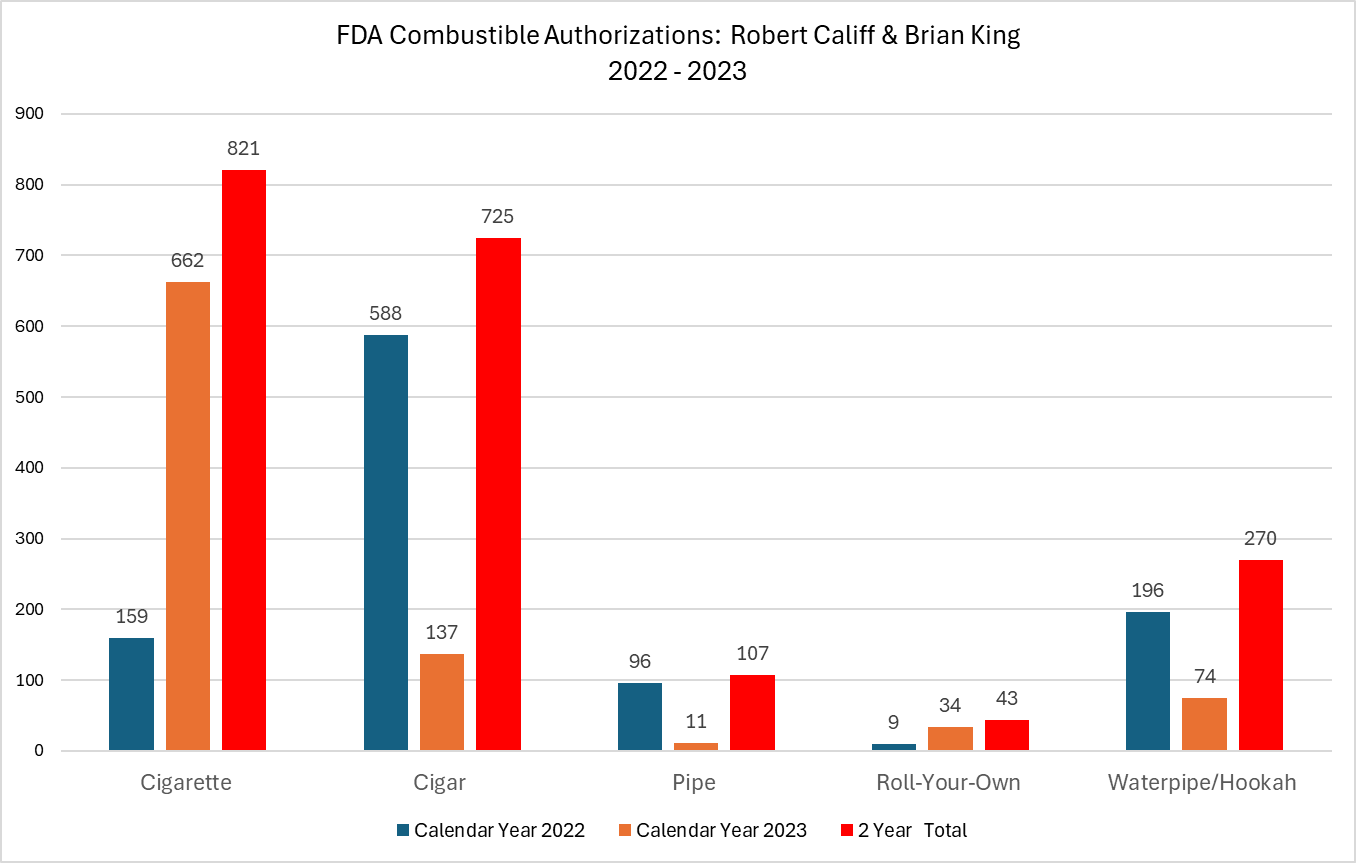
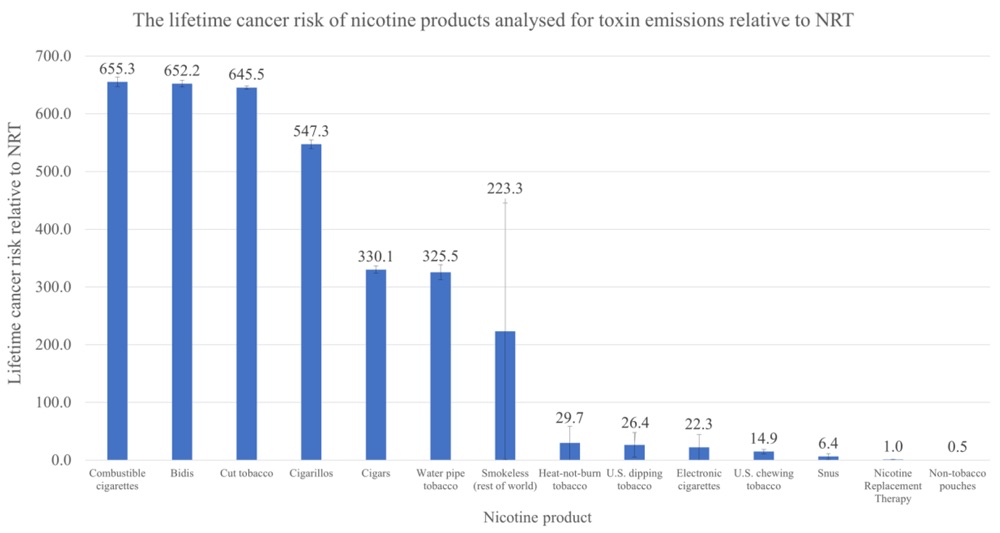
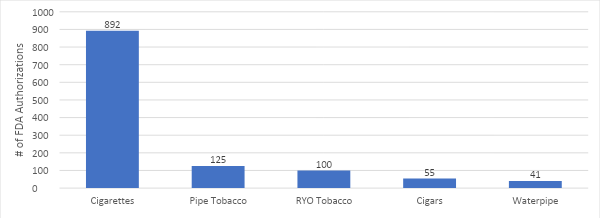
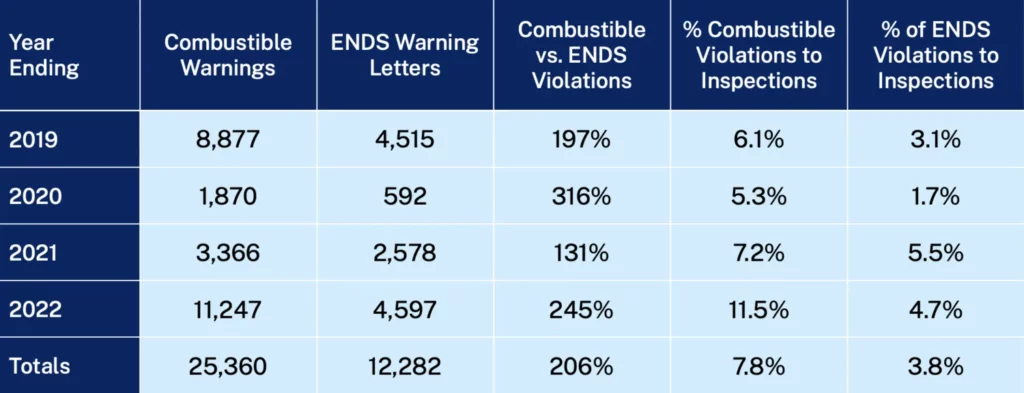
Introduction
This analysis provides an overview of the economic burden of cigarette smoking in the United States over the past 10 years, and examines evidence of healthcare cost and GDP benefits when smokers switch from combustible cigarettes to e‐cigarettes. The analysis draws on recent peer-reviewed studies and economic reports to quantify smoking-attributable healthcare expenditures, lost productivity, and potential cost savings from vaping as a less harmful alternative. All findings are cited to reputable sources for validation.
Healthcare Costs Attributable to Cigarette Smoking
A significant portion of U.S. healthcare spending is devoted to treating smoking-related diseases. By 2010, approximately 8.7% of all annual healthcare expenditures in the U.S. – about $170 billion – was attributable to cigarette smoking.[1] (Notably, over 60% of those medical costs were paid by public programs like Medicare and Medicaid[2]reuters.com, highlighting smoking’s burden on government health budgets.) These costs have grown over the past decade due to inflation and the compounding effects of chronic disease.
The CDC reported that in 2018 cigarette smoking cost the United States more than $600 billion in total, with more than $240 billion in direct health care spending on smoking-related medical conditions. This includes hospitalizations, medications, long-term care, and other treatments for diseases caused or exacerbated by smoking (such as lung cancer, chronic obstructive pulmonary disease, heart disease, and stroke). For example, smoking is a primary driver of chronic lung diseases – U.S. spending on chronic obstructive pulmonary disease (COPD) alone was about $34 billion in 2016, much of which can be linked to smoking.[3]
Per-capita healthcare costs for smokers are very high. The 2018 spending implies an average of roughly $6,000–$7,000 in annual medical costs per smoker (based on ~$240 billion across ~35 million smokers), far above the norm for non-smokers. These expenses include treating cancers, cardiovascular events, respiratory illnesses, and numerous other conditions caused by smoking. By contrast, smoking cessation or avoidance leads to rapid health improvements and cost reductions – within just a year of quitting, medical expenditure declines are measurable, and over a lifetime each quitter saves thousands in healthcare dollars.
Productivity Losses and GDP Impact of Smoking
Beyond healthcare outlays, smoking imposes enormous indirect costs on the economy through lost productivity. Smokers tend to have higher absenteeism and reduced on-the-job productivity, and many die prematurely during their prime working years. In 2018, lost productivity due to smoking (including work absences, reduced on-the-job performance, and premature mortality) was estimated at nearly $372 billion.[4] When combined with healthcare spending, the total economic cost of smoking exceeded $600 billion in 2018.[5] This figure is equivalent to a substantial portion of the U.S. economy – for context, one analysis found smoking-related costs amounted to approximately 4.3% of U.S. GDP in 2020.[6] In dollar terms, annual losses from smoking reached $891.8 billion in 2020 (including medical costs and productivity losses), about ten times the total revenue of the U.S. cigarette industry in that year.[7]
It is also useful to note the scale of the U.S. smoking burden relative to global figures. Worldwide, the economic cost of smoking has been estimated at about 1.8% of global GDP (roughly $1.4 trillion USD annually)tobaccocontrol.bmj.com. The fact that the United States incurs a cost on the order of 3–4% of its GDP from smoking underscores the outsized impact in this country, likely due to higher healthcare costs per person and higher incomes lost per smoker. In short, smoking has been a significant brake on U.S. economic productivity and growth in the past decade, manifesting in both direct medical expenses and the opportunity costs of a sicker, smaller workforce.
Switching from Smoking to Vaping: Health and Cost Implications
Given the enormous health and economic burden of smoking, there has been growing interest in harm reduction strategies – particularly the substitution of combustible cigarettes with electronic cigarettes (e-cigarettes). E-cigarettes (battery-powered devices that deliver nicotine via an aerosol vapor) are widely understood to be less harmful than smoking cigarettes, because they do not burn tobacco and thus produce far lower levels of harmful constituents. As early as 2015, Public Health England and other expert bodies have reported that vaping is around 95% less harmful than smoking in terms of long-term health risk.[8] While e-cigarettes may not be risk-free (they still deliver nicotine and some irritants), research consistently shows marked reductions in exposure to toxic substances and improved health indicators among smokers who completely switch to vaping. For example, as far back as 2018, the U.S. National Academies of Science concluded that complete switching from cigarettes to e-cigarettes results in substantially lower exposure to numerous toxicants and carcinogens, and smokers who switch experience improved respiratory and cardiovascular health markers in the short term.[9]
For these and many other reasons, leading U.S. tobacco control scientists have chastised the current U.S. regulatory approach of “ignoring” the benefits of vaping products or otherwise recognizing them as a key tool for smoking cessation. In February 2024, Dr. Nancy Rigotti, a member of the U.S. National Academies of Science, Engineering and Medicine, wrote an editorial in the New England Journal of Medicine, in which she declared, “It is now time for the medical community to acknowledge this progress and add e-cigarettes to the smoking-cessation toolkit….U.S. public health agencies and professional medical societies should reconsider their cautious positions on e-cigarettes for smoking cessation. The evidence has brought e-cigarettes to a tipping point. The burden of tobacco-related disease is too big for potential solutions such as e-cigarettes to be ignored.”[10]
Healthcare Cost Savings from Switching to E-Cigarettes
Because vaping leads to better health outcomes than smoking, smokers who switch to e-cigarettes can generate significant healthcare cost savings. Early economic data bear this out. In 2018, researchers estimated that the total healthcare expenditures attributable to e-cigarette use was only $2,000 in medical spending per year for each adult vaper on average[11] Notably, this per-person healthcare cost for vapers is much lower than the per-person cost for smokers, which as noted above can range roughly from $6,000 to $7,000 per smoker annually. In other words, a smoker incurs several thousand dollars more in medical costs each year than they likely would as a vaper. These savings arise from avoiding expensive treatments for smoking-induced conditions – for instance, a longtime smoker might require costly interventions (chemotherapy, surgeries, long hospital stays) for lung cancer or heart disease, whereas a comparable individual who switched to vaping would have a greatly reduced risk of ever developing those conditions.
Over the past decade, numerous studies have modeled the potential healthcare savings if smokers transition to vaping. One analysis projected that replacing cigarette smoking with vaping on a large scale would significantly reduce the incidence of costly chronic illnesses. Over a 10-year period, the U.S. could see 6.6 million fewer premature deaths and 86.7 million fewer life-years lost if cigarette use were largely replaced by e-cigarette use.[12] Each life-year saved corresponds to reduced treatment and hospitalization needs, meaning tens of billions of dollars in healthcare savings when extrapolated nationwide. Even partial switching can yield benefits: for example, if even 10% of smokers in the U.S. switched to vaping, that could avert a significant number of heart attacks, strokes, and cancer cases in coming years, translating into many billions of dollars saved in hospital care and chronic disease management.
GDP and Productivity Gains from Switching
In addition to direct healthcare savings, moving smokers onto less harmful e-cigarettes can confer wider economic benefits by improving productivity and reducing the drag on GDP. Healthier individuals are generally more productive workers: they take fewer sick days, are less likely to become disabled, and can contribute to the economy for more years. When smokers switch to vaping, their risk of debilitating illness or early death drops, meaning they are more likely to remain active in the workforce. Economists note that reaching national smoking reduction goals would “help divert scarce resources away from treating tobacco-related illnesses towards growing market productivity and household income.”[13] In other words, the money not spent on medical care can be invested in other sectors of the economy, and smokers who avoid disease can continue to work, earn, and spend, which stimulates economic activity.
The potential GDP impact of widespread switching is substantial. For context, smoking-related productivity losses (due to absenteeism and premature mortality) cost the U.S. around $185–$200 billion per year in recent estimates.[14] If a large fraction of smokers become vapers, a significant portion of this loss could be recouped. Even a mid-range harm reduction scenario might return tens of billions of dollars in annual productivity (from fewer sick days and longer careers). Over years, this translates into higher GDP growth than would occur if smokers continued to incur high illness and mortality rates. Nationally, analysts have suggested that tobacco harm reduction could avoid such large productivity losses that, cumulatively, the U.S. economy could be hundreds of billions of dollars stronger over the coming decades than it would be if current smokers do not switch.
Conclusion
Over the last decade, cigarette smoking has exacted a staggering toll on U.S. healthcare resources and economic productivity, costing on the order of $600–$900 billion per year in combined medical expenses and lost output. This drag on GDP underscores the urgency of reducing smoking prevalence. E-cigarettes have emerged as the dramatically less harmful alternative to combustible cigarettes, and a growing body of research indicates that substituting smoking with vaping can yield significant health care cost savings and economic gains. Smokers who switch to e-cigarettes not only reduce their risk of disease, but also reduce their medical expenditures – recent data show vaping-related health costs are only a fraction of smoking-related costs on a per-user basis. At the population level, transitioning smokers to less harmful e-cigarette use could save billions in healthcare spending by preventing disease, while also improving workforce productivity and boosting GDP by reducing smoking-related absences, disability, and premature deaths.
Thus, facilitating a shift from smoking to vaping (without introducing other tobacco risks) appears to be economically beneficial for the United States. The healthcare system would spend less on treating preventable tobacco illnesses, and employers and governments would benefit from a healthier, more productive population. While continued research is needed to monitor long-term outcomes of e-cigarette use, the evidence to date strongly supports that every smoker who switches to vaping is likely to generate measurable cost savings for society in addition to personal health benefits. Public health policymakers can no longer ignore the enormous public health and economic benefits of making e-cigarettes “part of the smoking cessation toolkit”.
[1] Annual Healthcare Spending Attributable to Cigarette Smoking: An Update, December 2014, American Journal of Preventive Medicine 48(3).
[2]Kennedy, Madeline, Cigarette smoking costs weigh heavily on the healthcare system, Reuters, December 19, 2014, Reuters. https://www.reuters.com/article/business/healthcare-pharmaceuticals/cigarette-smoking-costs-weigh-heavily-on-the-healthcare-system-idUSKBN0JX2BD/#:~:text=system%20www,in%20the%20American%20Journal
[3] Gu D, Sung HY, Calfee CS, Wang Y, Yao T, Max W. Smoking-Attributable Health Care Expenditures for US Adults With Chronic Lower Respiratory Disease. JAMA Netw Open. 2024 May 1;7(5):e2413869. doi: 10.1001/jamanetworkopen.2024.13869. PMID: 38814643; PMCID: PMC11140527.
[4] Centers for Disease Control and Prevention [CDC], https://archive.cdc.gov/www_cdc_gov/tobacco/data_statistics/fact_sheets/fast_facts/diseases-and-death.html
[5] Id.
[6] Nargis, N., et al. (2022) Economic loss attributable to cigarette smoking in the USA: an economic modelling study. The Lancet Public Health. doi.org/10.1016/S2468-2667(22)00202-X.
[7] Id.
[8] E-cigarettes: an evidence update, Public Health England, August 19, 2015, https://www.gov.uk/government/news/e-cigarettes-around-95-less-harmful-than-tobacco-estimates-landmark-review#:~:text=An%20expert%20independent%20evidence%20review,harmful%20to%20health%20than
[9] National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public Health Consequences of E-Cigarettes. Eaton DL, Kwan LY, Stratton K, editors. Washington (DC): National Academies Press (US); 2018 Jan 23. PMID: 29894118.
[10] Rigotti, Nancy, M.D., Electronic Cigarettes for Smoking Cessation – Have We Reached a Tipping Point?, New England Journal of Medicine, 390:7, February 15, 2024.
[11] Wang Y, Sung HY, Lightwood J, Yao T, Max WB. Healthcare utilisation and expenditures attributable to current e-cigarette use among US adults. Tob Control. Published online May 23, 2022. doi:10.1136/tobaccocontrol-2021-057058
[12] Levy DT, Borland R, Lindblom EN, et al. Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob Control. 2018;27(1):18–25. doi: 10.1136/tobaccocontrol-2017-053759
[13] Nargis, N., et al. (2022) Economic loss attributable to cigarette smoking in the USA: an economic modelling study. The Lancet Public Health, Volume 7, Issue 10, e834 – e843.
[14] See note 4.