“The removal of Brian King from a position of leadership impacting millions of Americans is the first step in correcting the broken mindset that has crippled the FDA and the Center for Tobacco Products over the past four years. The next step is fixing the broken regulation that allowed CTP, under King’s leadership, to make politically motivated decisions that have done nothing to save the 480,000 Americans who die from smoking every year. Instead of following the science, as was his mandate, he chose to ignore it, standing in the way of President Trump’s campaign promise to save flavored vaping.
VTA applauds President Trump and Secretary Kennedy for taking decisive action and looks forward to working together to ensure Americans have access to less harmful flavored vaping products and that America is Made Healthy Again.”
VTA Response to SCOTUS Ruling on FDA v. Wages & White Lion Investments
WASHINGTON – April 2, 2025 – The following statement is attributable to Tony Abboud,
Executive Director of the Vapor Technology Association:
“We are deeply disappointed by the Supreme Court’s ruling today in FDA vs. Wages & White Lion Investments. The decision is an endorsement of the FDA’s shifting conduct, and we strongly disagree with its implications for the vaping industry and the millions of adult consumers who rely on flavored products as a harm reduction tool.
This ruling underscores the urgent need for decisive action from the Trump Administration to resolve ongoing – and future – litigation by eliminating once and for all the regulatory uncertainty plaguing the FDA. President Trump’s FDA can establish clear and fair guidance that supports innovation and ensures the survival of the small businesses that make up the U.S. vaping industry.The future of flavored vaping now lies in President Trump’s hands, and we urge him to fulfill his campaign promise to save flavored vaping – the most effective tool available to quit smoking deadly cigarettes.
We remain committed to advocating for sensible policies that balance public health priorities with consumer choice and industry sustainability and look forward to working with President Trump to make these changes – now more necessary than ever – a reality.”
Vapor Technology Association Launches Broadcast Campaign Calling on President Donald Trump to Keep Promise to Save Flavored Vaping Industry
The broadcast segments feature small business owners and vaping voters relying on President Trump to save the vaping industry.
Washington, D.C. – Wednesday, March 19, 2025: Today, The Vapor Technology Association (VTA) launched a cable and digital news campaign urging President Donald Trump and his administration to uphold their promise to save the flavored vaping industry and the thousands of small businesses the industry supports. The weeklong placements are running on FOX News, FOX Business, and Newsmax during key programming slots, including “FOX and Friends,” “Kudlow,” and “The Five.”
VTA’s campaign emphasizes the trust American vapers have placed in President Trump to protect small businesses and consumer choice. The segments feature small business owners and dedicated vaping supporters who stood up for President Trump during the 2024 election and are now asking him to stand up for them.
“In September, President Trump made a promise on his Truth Social account that he would save the flavored vaping industry, protect tens of thousands of small businesses from being shut down, and protect the tens of millions of adult Americans who smoke but need flavored vaping alternatives to quit,” said Tony Abboud, executive director of VTA. “Vaper voters, who turned out by the hundreds of thousands to support then-candidate Trump, have given President Trump a mandate to keep the promise he made and protect the thousands of small businesses and Americans that rely on vaping products.”
The placements also highlight the consequences of inaction on the issue, including the destruction of a multibillion dollar industry.
To watch VTA’s “Save My Small Business” broadcast placement, please visit this link.
# # #
About VTA: The Vapor Technology Association is the U.S. industry trade association whose members are dedicated to sound science-based regulation and selling innovative high-quality vapor products that provide adult smokers with a better alternative to combustible cigarettes. VTA represents the industry-leading manufacturers of vapor devices, e-liquids, and flavorings, as well as the distributors and retailers, including hardworking American mom-and-pop brick-and-mortar retail store owners.
ICYMI: “PROMISES MADE; VAPES KEPT” LAUNCHED WITH PLACEMENT ON FOXNEWS, CONSERVATIVE TV URGING PRESIDENT TRUMP TO SAVE FLAVORED VAPING
Washington, D.C. – Thursday, January 23, 2025: The Vapor Technology Association (VTA)
recently launched a significant “Promises Made; Vapes Kept” broadcast placement and ad
campaign on Fox News urging President Trump and his administration to uphold their campaign
promise to save the flavored vaping industry and the thousands of small businesses the industry
supports. The weeklong broadcast television spot on Fox News started running immediately
following the historic second inauguration of President Trump reaching viewers in Washington,
D.C. and nationwide.
“In September, President Trump made a promise on his Truth Social account that he would
save the flavored vaping industry, protect tens of thousands of small businesses from being
shut down, and protect the tens of millions of adult Americans who smoke but need flavored
vaping alternatives to quit,” said Tony Abboud, executive director of the Vapor Technology
Association. “VTA, vaper voters, and small vape businesses turned out by the hundreds of
thousands to support then-candidate Trump and help him secure his electoral victory. VTA
looks forward to working with President Trump to fulfill his promise to save the flavored vaping
industry in his first 100 days as president.”
VTA’s broadcast placement is running on Fox News, Fox Business, and Newsmax, from
January 21 to January 27, 2025. Programs included, but were not limited to, Fox & Friends, The
Five, Hannity, Mornings with Maria, Kudlow, and more.
VTA Files SCOTUS Amicus Brief Opposing FDA in Wages & White Lion Case

Washington, DC – October 15, 2024 – The Vapor Technology Association (VTA) filed with the Supreme Court of the United States (SCOTUS) an amicus brief against the FDA and in support of Respondents in United States Food & Drug Administration v. Wages & White Lion Investments, dba Triton Distribution et al.
Unlike the amicus briefs we filed in this on-going litigation which focused on the FDA’s wrongdoings and the adverse economic impact of failing to hold the FDA to legal account, this brief focused on the recent dramatic change in SCOTUS jurisprudence in Loper Bright v. Raimondo which dramatically altered the manner in which federal courts evaluate whether agencies – like the FDA – have acted within the confines of the law by assessing the best reading of the statute.
VTA’s amicus brief supports Respondents arguments and encourages SCOTUS to look skeptically – through the prism of Loper – at the FDA’s arguments and its post-hoc justifications for its actions. VTA’s amicus brief highlights three key points:
- LOPER REQUIRES THAT FDA’S DENIAL ORDERS BE SET ASIDE BECAUSE THEY VIOLATE THE BEST READING OF THE TOBACCO CONTROL ACT.
- THE BEST READING OF THE TOBACCO CONTROL ACT DOES NOT ALLOW FDA TO ISSUE DENIAL ORDERS BASED ON THE POST-HOC COMPARATIVE EFFICACY TEST.
- FDA PROCESS FOR REVIEWING PMTAS WAS HEAVILY CRITICIZED BY AN INDEPENDENT TOBACCO EXPERT PANEL WHICH CALLED OUT THE FDA’S FAILURE TO CONSISTENTLY APPLY THE APPH TEST.
VTA’s full amicus brief is available HERE.
The FDA is Objectively Failing
With over 16 million Americans suffering from smoking related disease, and nearly 500,000 Americans dying annually, the FDA’s Center for Tobacco Products has chosen to use its enormous regulatory power to deprive Americans of access, if not outright ban, all non-combustible alternatives to cigarettes in the U.S. despite leading tobacco control researchers demonstrating they are the most effective tool available to help people quit smoking.
“It is now time for the medical community to acknowledge this progress and add e-cigarettes to the smoking cessation toolkit… U.S. public health agencies and professional medical societies should reconsider their cautious positions on e-cigarettes for smoking cessation. The evidence has brought e-cigarettes to the tipping point. The burden of tobacco-related disease is too big for potential solutions such as e-cigarettes to be ignored.”
Dr. Nancy Rigotti, Harvard Medical School
Only a few years ago – regulators, scientists and harm reduction advocates arrived at a policy consensus that would balance the relative risks posed by various tobacco products. That consensus was driven by shared goals – to reduce the number of Americans who smoke cigarettes and move them down the “continuum of risk” away from combustible tobacco and toward less risky alternatives especially vaping and other non-combustible nicotine products. The most recent government data that tracks progress in this regard is unfortunately telling us that FDA policies are risking the lives of many Americans – especially older Americans who are most at risk from continuing this deadly habit.
In August of 2021, the 15 past presidents of the staunchly anti-tobacco Society for Research on Nicotine and Tobacco published a groundbreaking essay in the American Journal of Public Health. In it, they encouraged a more balanced consideration of vaping and other alternative nicotine products within public health and in the media and policy circles. While acknowledging that vaping is not free from risks, they demonstrated that it is substantially less harmful than cigarette smoking. Furthermore, they highlighted emerging evidence showing that vaping can increase smoking cessation and is likely more effective than FDA-approved nicotine replacement products like gum and patches.
Two years later, the FDA echoed some of those same observations and recommendations. In an August 2023 commentary published in the online journal Addiction and authored by Brian King, the current head of FDA’s Center for Tobacco Products (CTP), King highlighted that many adults were misinformed about the relative risks of various tobacco products and that misunderstanding was a barrier to moving smokers down the continuum of risk toward safer, non-combustible tobacco products. And yet, the CTP during his tenure has authorized a mere handful of non-combustible nicotine products. Instead, CTP has authorized thousands of new combustible tobacco products. As a result, millions of Americans continue to smoke and die from tobacco related disease.
American Smoking Deaths Under the Califf/King FDA
Since February 17, 2022
830,000,000
For an agency that spends a great deal of time talking about saving lives, by every empirical measure, they are objectively failing:
- During the period that Brian King and FDA Chief Robert Califf have been in charge of tobacco policy for the country, FDA has authorized more than 1,500 new cigarettes and more than 11,000 combustible tobacco products
- According to the 2023 National Youth Tobacco Survey (NYTS), past 30-day cigarette use among 12th graders, INCREASED from 5.7% to 7.5%
- The independent Reagan-Udall Foundation criticized the FDA for putting politics over science and ignoring emerging evidence that confirms that flavor bans and other prohibitions on non-combustible nicotine products actually increase cigarettes sales
- FDA’s own tobacco enforcement statistics confirm that smoking remains a problem and that youth smoking violations are higher than youth vaping
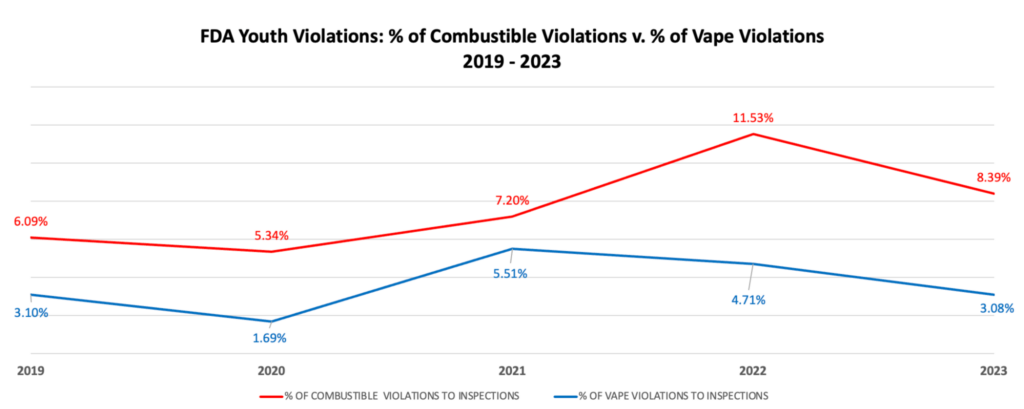
Scientists are now starting to sound the alarm and are warning that we have reached a tipping point where FDA’s broad opposition to flavored non-combustible tobacco products over the last four years will create enormous public health consequences in the future for many Americans.
Dr. Nancy Rigotti, Professor of Medicine at Harvard Medical School and a member of the National Academies of Science Engineering and Medicine, has sounded the alarm after publishing a editorial stating that we have reached “the tipping point” where policymakers can no longer willfully ignore evidence and use their regulatory authority to undermine e-cigarettes.
“It is now time for the medical community to acknowledge this progress and add e-cigarettes to the smoking cessation toolkit… U.S. public health agencies and professional medical societies should reconsider their cautious positions on e-cigarettes for smoking cessation. The evidence has brought e-cigarettes to the tipping point. The burden of tobacco-related disease is too big for potential solutions such as e-cigarettes to be ignored.”
FDA now finds itself in the awkward position of being a health agency that is facilitating the sale of cigarettes thereby undermining its own mission to reduce the incidence of smoking related disease and death. FDA’s policies are keeping more effective (and safer) alternatives to cigarettes away from consumers and are in turn propping up the sales and profits of big tobacco companies. Without a course correction that truthfully portrays the risks and benefits of various nicotine products, millions of Americans will needlessly suffer from illness and death.
VTA Insider June 2024: VTA Testifying before congress, Update on the state of States
Welcome to the VTA Insider, your industry focused briefing from the Vapor Technology Association.
The VTA Insider will keep you informed of critical issues impacting the vaping industry both in Washington, DC and in the states. We will cover regulatory developments, legislation, news of note and other industry insights. VTA will also share with you our own analysis of what we as an industry need to do to grow and prosper.
Lets Dive In.
VTA testifies before Congress
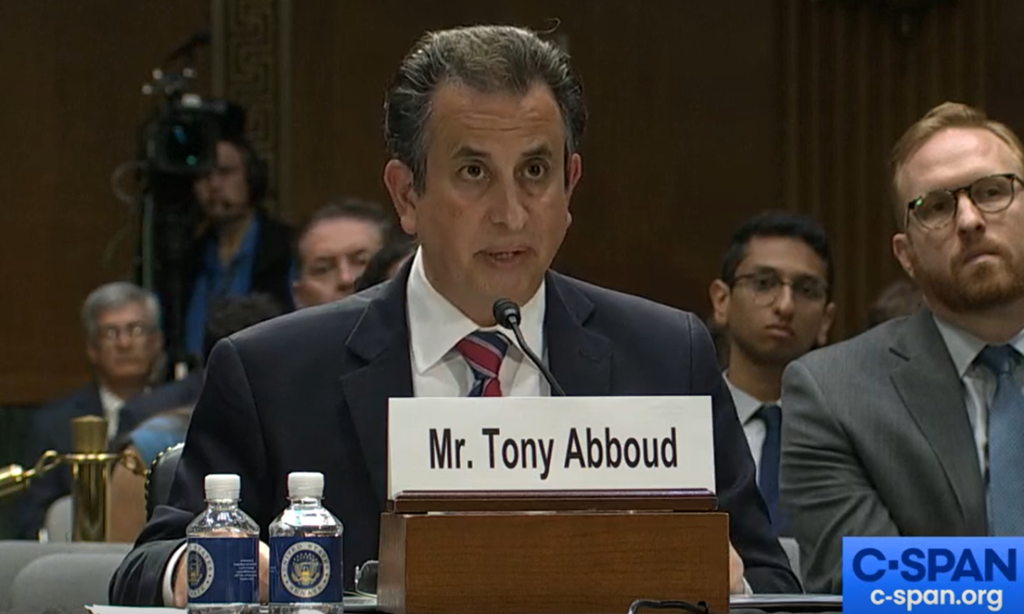
On Wednesday, June 12, 2024, VTA’s Executive Director Tony Abboud had the unique opportunity to testify before the Senate Judiciary Committee in Washington, D.C. to address the myriad problems with FDA’s regulatory approach to e-cigarettes. Abboud’s testimony provided the Committee with critical scientific data shared tobacco-control scientists’ calls for a dramatic change in U.S. regulators’ approach to e-cigarettes.
What we’re saying: Abboud’s testimony touched on three key points:
- FDA’s many failures and the hypocrisy of the leading health regulator authorizing 821 new cigarettes and ZERO less harmful vaping or pouch products in the last two years.
- The facts regarding the dramatically ameliorated youth vaping rates in the U.S. coupled with an urgent message to Congress to take up the long overdue marketing and access restrictions that VTA has been advocating for since 2018 to actually protect youth.
- A renewed our call for the FDA to reverse course, authorize a wide variety of flavored e-cigarettes, make harm reduction its north star to encourage Americans who smoke to try e-cigarettes, and to enforce the Tobacco Control Act in a manner that will give tens of millions of Americans a regulated choice of less harmful flavored nicotine options and a fighting chance to get off deadly cigarettes.
Go deeper: You can catch the full hearing testimony here and read a transcript of Mr. Abboud’s opening testimony here.
UPDATED: State of the states
State of the States: Most state legislative sessions have adjourned till the fall, but there are still a few PMTA registry bills working their way through:
- In North Carolina, PMTA registry language was snuck into H900 without any hearing or public input on the matter by big lobbyists activists. If you live / work in North Carolina, please use and share our call to action to help stop this pernicious and desperate tobacco ploy today. Your representatives need to hear from you.
- In Louisiana, HB 621 updating the state’s PMTA registry bill passed both houses of the state legislature and was signed by Gov. Jeff Landry.
- In Rhode Island. Gov. Dan McKee signed the state budget bill H 7225, converting the health department’s flavor ban to a state law.
- In Iowa, Gov. Kim Reynolds signed HF 2677 into law, beginning the process of creating a PMTA registry in Iowa later this fall.
What’s next: Our fight against these bills is not over.
- We expect a few more states to address PMTA registration bills this year and we expect new and renewed attempts to create or expand PMTA registries next year.
- VTA is hard at work educating on the public health and small business damage these bills would create.
Vaping and Chevron deference

This week or next, the United States Supreme Court will release its remaining opinions of the term. The Court typically waits until final week to release to “big” decisions and there is none bigger than Loper Bright v. Raimando, the case that challenges the long standing doctrine known as Chevron deference.
Why it matters: Chevron deference provides federal agencies with the ability to interpret and implement ambiguous statutes, so long as these interpretations are “reasonable.” For years, the doctrine has been used to uphold federal agency actions even when they seem to exceed statutory authority.
For the vaping industry that has meant:
- FDA’s arbitrary and capricious vaping policies have been protected by Chevron deference.
- The vaping industry has been forced to bow to the political whims of an agency that does not want to approve less harmful non-combustible nicotine products.
What’s Next: Some legal analysts have opined that the Supreme Court could overturn or severely restrict Chevron deference and others have opined that the Court could narrowly restrict its ruling to the facts of the case.
- If the Court does the former, we see an avenue to force the FDA to fix their infamous “fatal flaw” approach that was designed to quickly deny marketing authorization for as many non-tobacco flavored ENDS as possible and start relying on science instead of politics in the PMTA process.
Flavored vapes for thee, but not for me

Last week, in a first, the FDA authorized menthol flavored vaping devices and finally acknowledged the enormous body of science proving flavored e-cigarettes help Americans quit smoking – not to mention FDA’s first authorization for a flavored disposable product at the highest nicotine concentration available on the market.
- The catch is that, to date, the only vaping products authorized are those manufactured by Big Tobacco companies.
- The second catch is that the week prior, FDA denied several other applications from small vaping maufacturers and wants to keep their products off the market.
Why it matters: While the news is a tiny step in the right direction, it also reveals a more troubling pattern:
- FDA is acting only in self-interest to quell political pressure rather than acting in the interest of the American people.
- Approving some but denying other substantially similar products is going to bring renewed scrutiny on how FDA is arbitrarily applying its it’s public health standard which, to date, it has refused to clearly articulate.
The bottom line: The only vapes authorized today are all owned by the biggest cigarette companies in the world, demonstrating Brian King and the FDA’s hypocritical allegiance to those companies whose deadly cigarettes and other combustible products the FDA continues to flood the market with at a record pace (more below).
E-cigarettes are the most effective smoking cessation tool on the market and are at least 95% safer than combustible cigarettes. It is far past time for this FDA to approve a wide variety of flavored e- cigarettes and stop authorizing dangerous cigarettes.
FDA’s tawdry Big Tobacco record
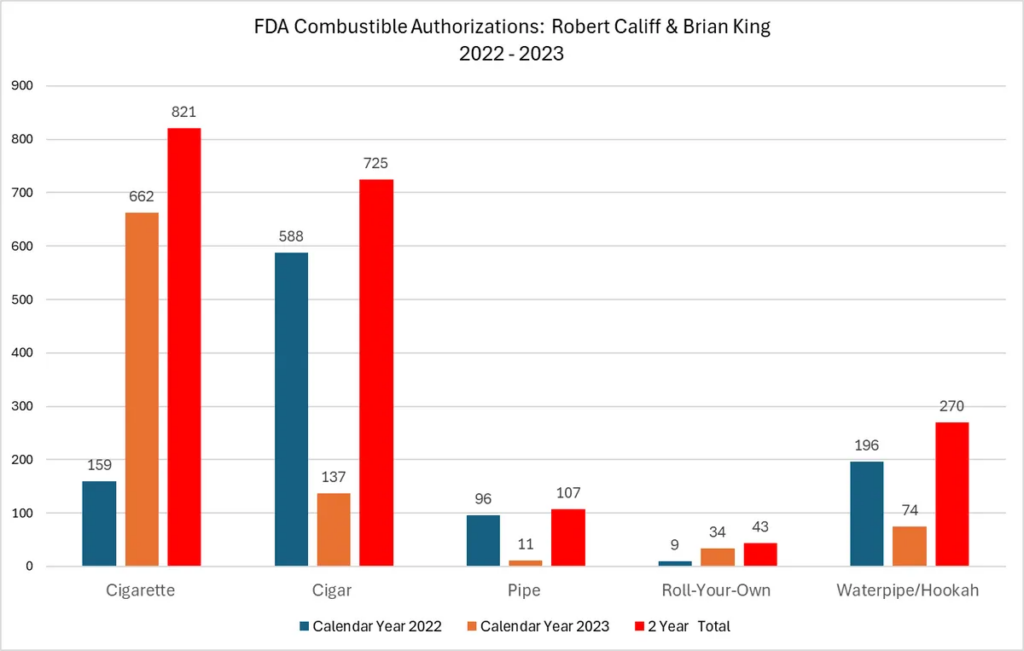
Last year, VTA did a deep dive into FDA’s tobacco product approvals. The numbers were shocking then, and things have only gotten worse as our updated May 2024 report FDA is on Fire proves.
Why it matters: Until last week, FDA has refused to authorize a single premarket tobacco application for less harmful electronic nicotine delivery systems. And they have refused to authorize a single less harmful modern oral nicotine product.
What we found: In stark contrast to the more than 16,800 combustible products that FDA has authorized from 2009 to December 2023, the FDA has seen fit to authorize a paltry 45 next generation tobacco products. By category, FDA’s 45 authorizations include:
- 2 new combustible cigarettes (low nicotine, but still combustible)
- 4 oral tobacco mints and chews
- 8 smokeless tobacco products (snus)
- 8 heat-not-burn products (the IQOS device and assorted Marlboro HeatSticks)
- 23* e-cigarettes (out of more than 20M applications filed).
*Since facts do matter, it is important to note that the often-cited list of “23” authorized e-cigarettes, only includes about 8 unique e-cigarette devices (some of which are no longer available on the market). The remainder of the list of 23 “e-cigarettes” are either replacement pods, accessories, or other products not traditionally identified as e-cigarettes.
You can read VTA’s Report – FDA is on Fire here.
Post script: With the new menthol authorizations, the net available vape devices on the U.S. market now hovers around 10.
The FDA enforcement trap

The latest: At last month’s Food and Drug Law Institute’s Tobacco & Nicotine Policy Conference in Washington, D.C., VTA Executive Director Tony Abboud presented thoughts on FDA’s broken enforcement policies and what that means for the future of tobacco product regulation.
Go Deeper: Tony’s presentation highlighted some of the major problems that that FDA has created with its misguided policies in pretending that it can enforce prohibition:
- FDA policies have created a de facto ban on ENDS products resulting in ZERO product approvals during the current commissioner’s tenure;
- FDA’s enforcement of ENDS devices is not viable or efficient and is helping to create illicit markets;
- FDA’s enforcement policies are misplaced given that there are three times the number of youth violations for combustible tobacco products vs. vaping products;
- FDA’s flavor ban enforcement is driving smokers back to cigarettes
You can view a copy of Tony’s presentation here.
Worth Your Time

- VTA Executive Director Tony Abboud joins Fox News to discuss FDA’s failures and need for flavored e-cigarettes
- VTA Executive Director Tony Abboud quoted in Washington Post story
- Vape bans could lead to smoking comeback among children
- Phillip Morris suspends Zyn online sales nationwide after DC subpoena
Stay tuned in with us for lots of new developments and make sure your friends and colleagues are signed up to receive our news and information.
Got questions? If you have any questions about the issues we have covered, have suggestions for content, or how you can support our efforts, please feel free to contact us at press@vaportechnology.org.
VTA’s Executive Director Testifies Before Congress
VTA’s Executive Director testified before the Senate Judiciary Committee in a hearing focused on FDA’s tobacco market regulatory failures. Watch the full video here.
In his testimony, Tony highlighted how FDA’s failed approach causing real harm to millions of Americans.
“The FDA has not done its job as a regulator. And now it wants more funding to enforce the very actions which federal courts have declared as illegal while the FDA is asking for Supreme Court review. To be clear, the FDA has repeatedly stated that every single vaping product on the market is illegal regardless of whether the company has a pending application. That means FDA is enforcing prohibition. You can give it all the enforcement power in the world, but prohibition cannot be enforced.
Instead of pursuing enforcement action based on failed regulatory policy, the FDA must reverse course, make harm reduction its north star and authorize a wide variety of less harmful nicotine products. Filling the market with such flavored products which American adult consumers are clearly demanding is the only way curb illicit demand and give Americans what they need, want, and what the law intended them to have.”
Read a transcript of the testimony here.
VTA Report: FDA’s Tawdry Record With Cigarette Approvals
FDA has authorized more than 1,500 new cigarettes and more than 11,000 combustible tobacco products over the last five years.
Last year, VTA did a deep dive into FDA’s tobacco product approvals. The numbers were shocking then and things have only gotten worse.
FDA’s current leaders, Commissioner Robert Califf and Center for Tobacco Products Director Brian King, have refused to authorize a single premarket tobacco application for less harmful electronic nicotine delivery systems (ENDS) out of millions of products for which PMTAs were submitted. And they have refused to authorize a single less harmful modern oral nicotine product.
At the same time, under their combined leadership, Califf and King have authorized nearly 2,000 combustible tobacco products, of which 821 were new cigarettes with 662 new cigarettes being authorized in 2023 alone!
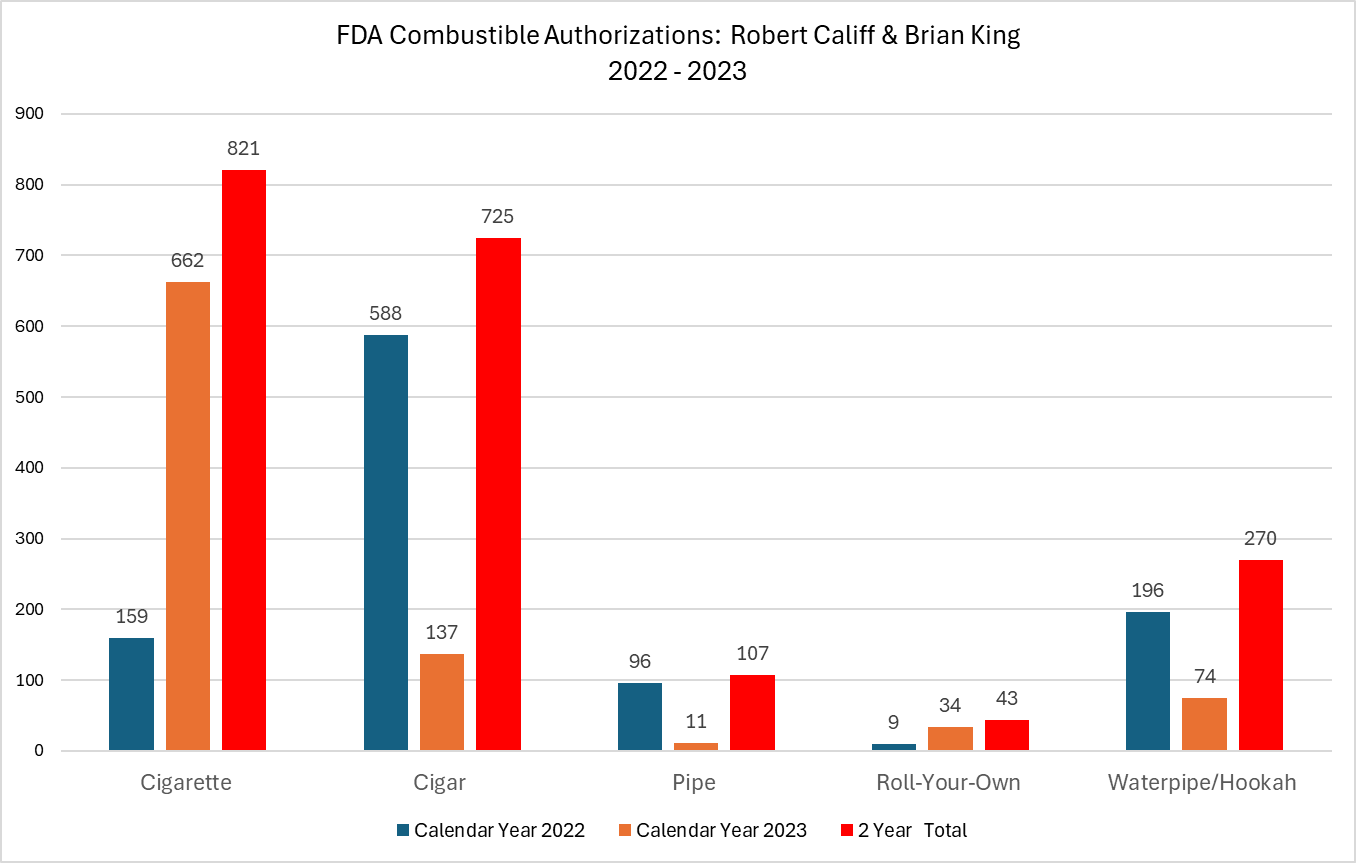
The FDA Commissioner actually testified last month that “the best thing to do is to stop using tobacco products altogether. The second best would be to switch to a vape.” Quite difficult to do when the agency he runs is singularly focused on pumping more and more new combustible tobacco products into the market while doing everything it can to rip vapes out of the hands of Americans trying to quit smoking.
So, what should we make of an agency which has become prolific in publicizing all of its anti-vaping actions while it is quietly accelerating deadly cigarettes into the market with rubber-stamp approvals?
Our new report – FDA Is On Fire – reveals FDA’s historical and current tawdry relationship with cigarettes and other combustible products and reveals that it has either lost sight of, or has completely abandoned, its primary mission of eliminating smoking.
Also Read: FDA Approves Combustible Tobacco at a Dangerous Rate
VTA’s Executive Director Addresses the ramifications of the FDA Commissioner’s testimony
VTA’s Executive Director Tony Abboud sat down with Regulator Watch to address the aftermath of the FDA Commissioner, Robert Califf’s testimony in front of the U.S. House Committee on Oversight and Reform . Watch the full video below.
- 1
- 2
- 3
- …
- 11
- Next Page »