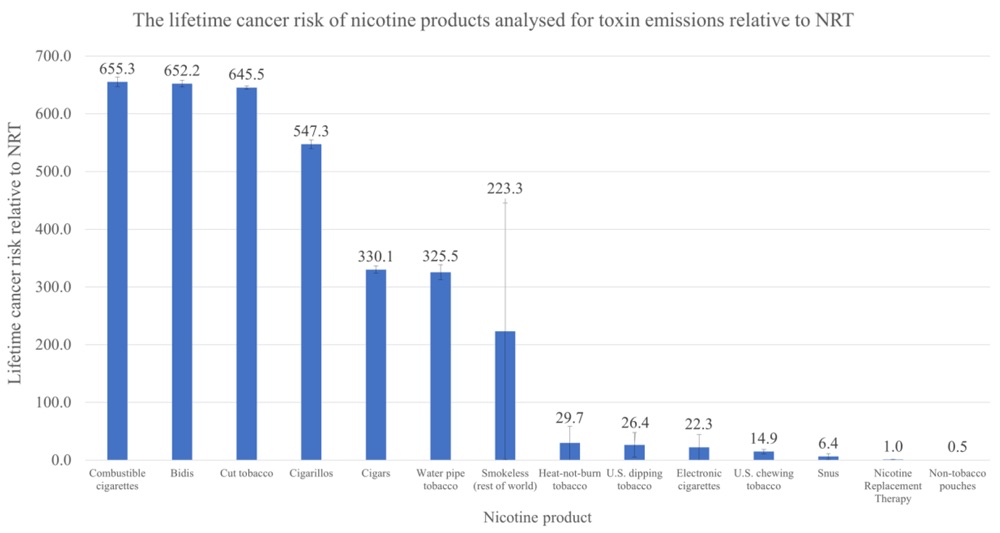
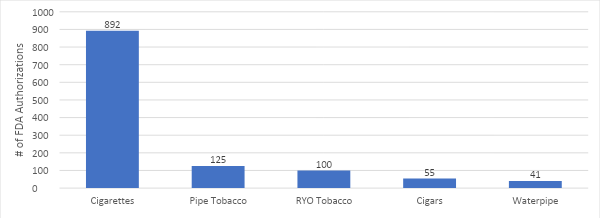
VTA’s Executive Director Tony Abboud sat down with Regulator Watch to unpack the results of the 2023 National Youth Tobacco Survey and what it means for the FDA. Watch the full video below.
Also Read Unraveling FDA Decisions: A Threat to Public Health or Playing Favorites?