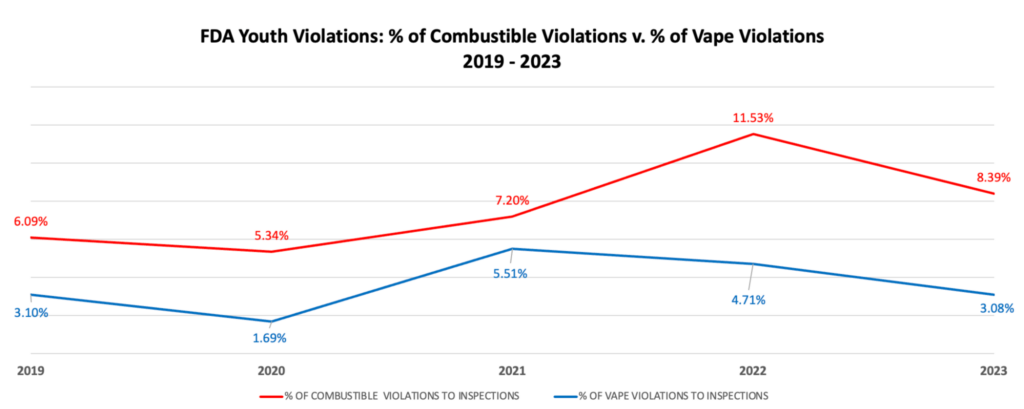
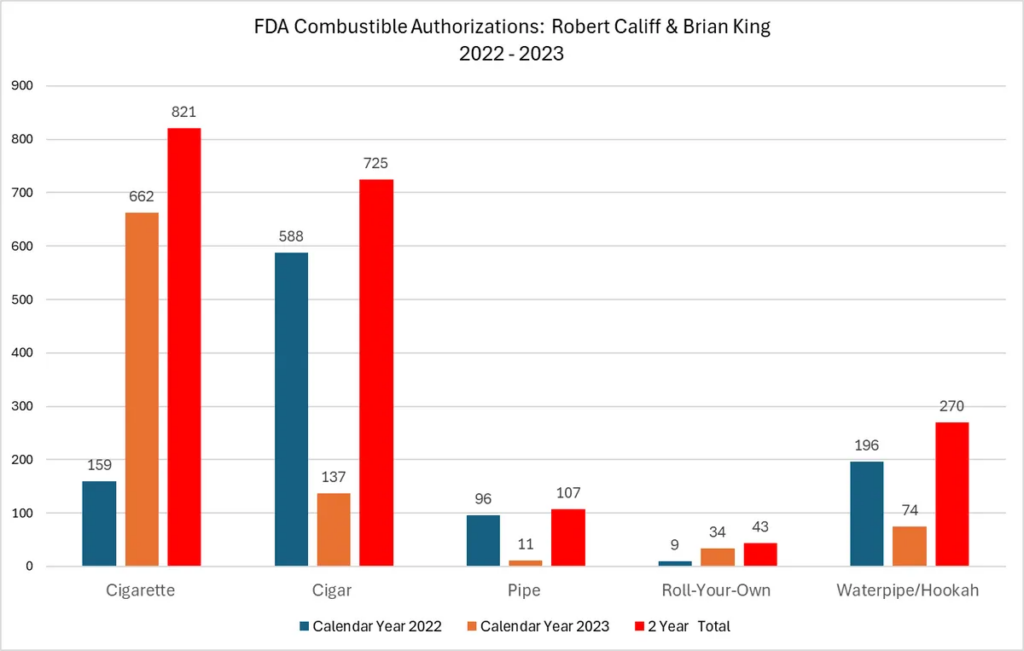
American-made vaping products—produced by companies that have fully complied with the FDA’s own guidance and the PMTA process—are now being seized nationwide. For more than five years, VTA has warned the agency of flaws in its regulatory system, yet the FDA refuses to engage with stakeholders—an irresponsible course of action that now threatens the bankruptcy of hundreds of thousands of small businesses nationwide.
As of today, the Secretary of Health and Human Services and the U.S. Attorney General are holding a press conference at a vape distribution center in Illinois to highlight their seizures. In Illinois alone, the vape industry produces an economic output of over $544 million and supports 2,666 jobs. Vapor businesses have paid over $175,998,400 in wages and benefits to their employees in the state and generated $92,383,700 in state and local tax revenue.
If this course of action continues, it will immediately wipe out 150,000 American small businesses and eliminate 90,000 jobs across the country. The result: a catastrophic loss of over $5 billion in state, local, and federal tax revenue this year alone—ballooning to $20 billion over the course of President Donald Trump’s second term. The vaping industry currently contributes more than $17 billion annually to the U.S. economy, and that economic engine will vanish overnight.
The consequences of this reckless course of action cannot be overstated. This is not only an assault on public health—it is an assault on American workers, small businesses, and the tax base that supports our communities.
The actions of HHS and the DOJ are detrimental to the state, local, and federal economies, and they must reverse course immediately.
Statement attributable to Tony Abboud, Executive Director of the Vapor Technology Association.