VTA’s Executive Director testified before the Senate Judiciary Committee in a hearing focused on FDA’s tobacco market regulatory failures. Watch the full video here.
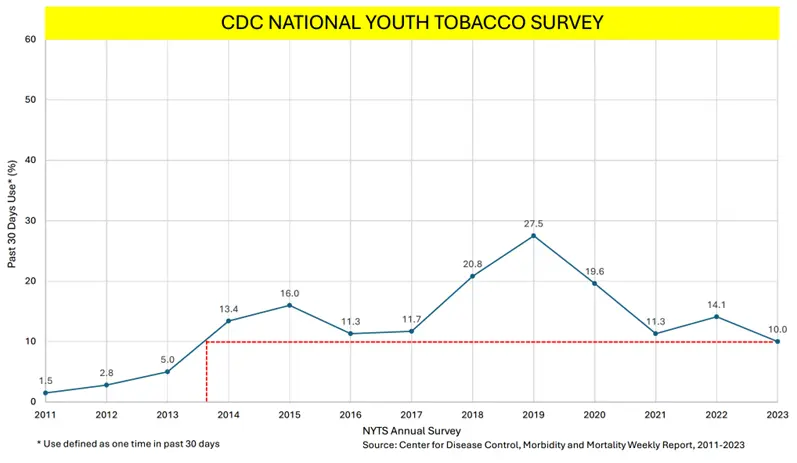
In his testimony, Tony highlighted how FDA’s failed approach causing real harm to millions of Americans.
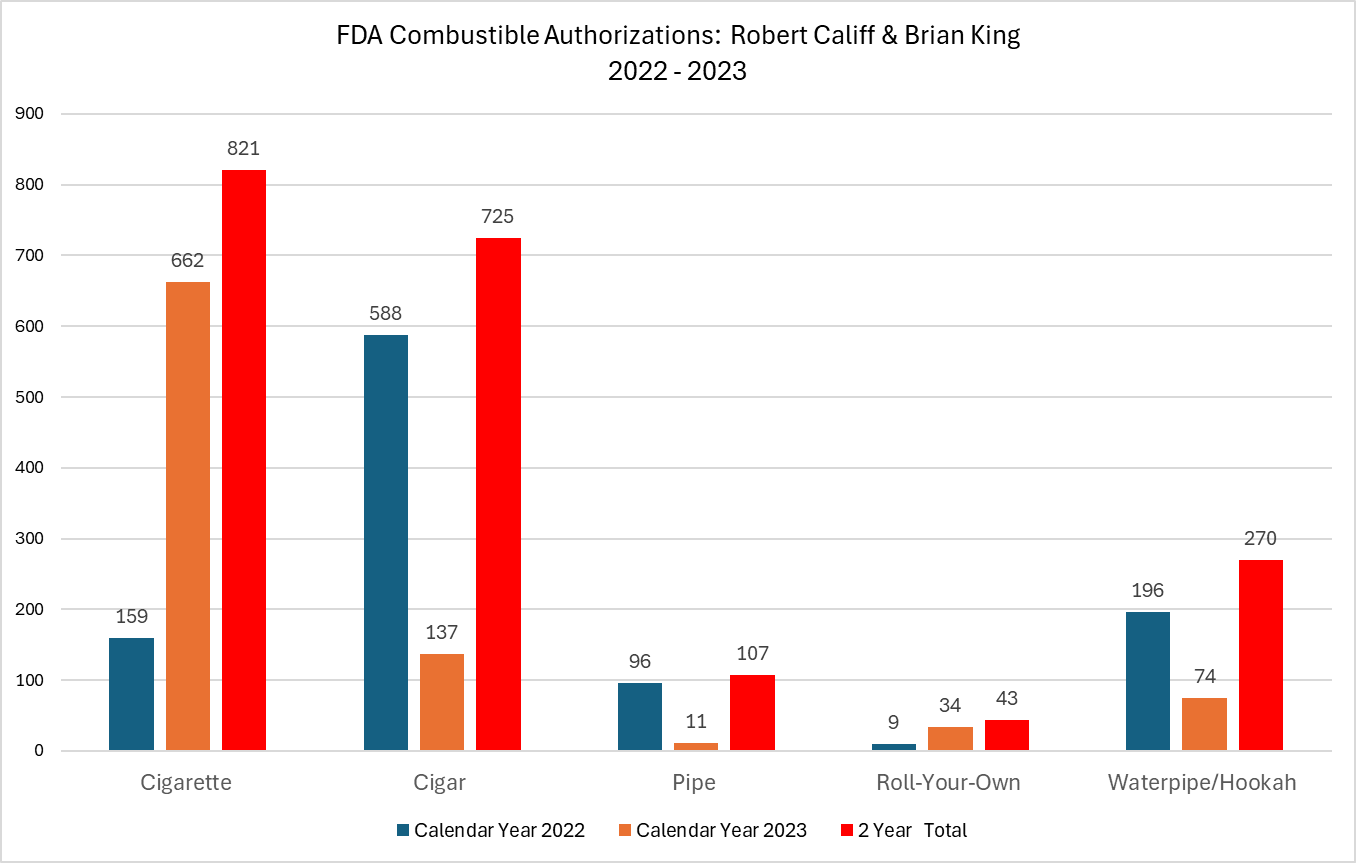
“The FDA has not done its job as a regulator. And now it wants more funding to enforce the very actions which federal courts have declared as illegal while the FDA is asking for Supreme Court review. To be clear, the FDA has repeatedly stated that every single vaping product on the market is illegal regardless of whether the company has a pending application. That means FDA is enforcing prohibition. You can give it all the enforcement power in the world, but prohibition cannot be enforced.
Instead of pursuing enforcement action based on failed regulatory policy, the FDA must reverse course, make harm reduction its north star and authorize a wide variety of less harmful nicotine products. Filling the market with such flavored products which American adult consumers are clearly demanding is the only way curb illicit demand and give Americans what they need, want, and what the law intended them to have.”
Read a transcript of the testimony here.